Complete the 𝐾a1 expression for h2co3 in an aqueous solution. – Complete the Ka1 expression for H2CO3 in aqueous solution. The Ka1 value, or acid dissociation constant, is a crucial parameter in understanding the acidity of a solution and the behavior of weak acids like carbonic acid (H2CO3). This article delves into the concept of Ka1, its significance, and the complete Ka1 expression for H2CO3 in aqueous solution, providing a comprehensive overview of this fundamental aspect of acid-base chemistry.
Ka1 is a measure of the extent to which an acid dissociates in water, indicating the strength of the acid. Factors like temperature, solvent, and ionic strength influence the Ka1 value. Understanding Ka1 is essential for various applications, including pH calculations, carbon dioxide sequestration, and water treatment.
Acid Dissociation Constant (Ka1): Complete The 𝐾a1 Expression For H2co3 In An Aqueous Solution.
The acid dissociation constant (Ka1) is a quantitative measure of the strength of an acid in a solution. It represents the equilibrium constant for the dissociation of the acid into its conjugate base and hydrogen ions (H+).
The value of Ka1 is affected by several factors, including temperature, solvent, and ionic strength. A higher Ka1 value indicates a stronger acid, as it dissociates more readily in solution.
Ka1 Expression for H2CO3
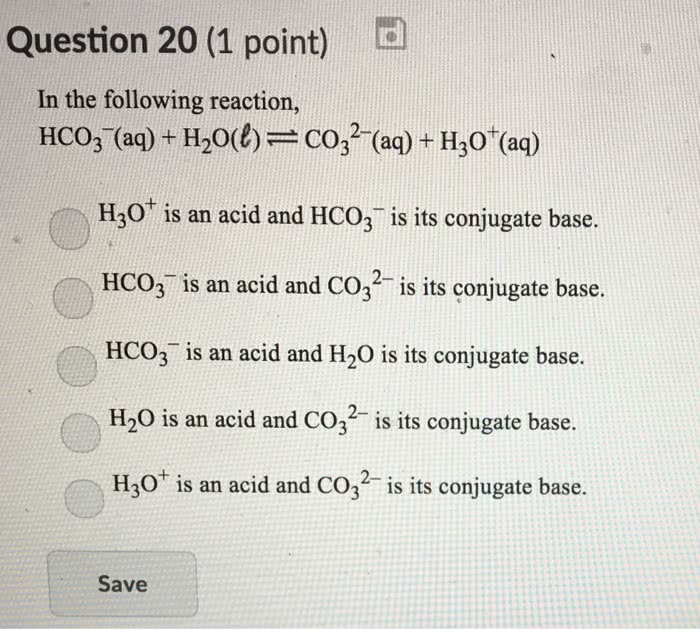
Carbonic acid (H2CO3) is a weak acid that dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-).
The Ka1 expression for H2CO3 in an aqueous solution is:
Ka1 = [H+][HCO3-] / [H2CO3]
where [H+], [HCO3-], and [H2CO3] represent the molar concentrations of the respective species in equilibrium.
Determining Ka1 Value

The Ka1 value of H2CO3 can be determined experimentally using various methods, such as potentiometry or spectrophotometry.
Potentiometry involves measuring the pH of a solution containing H2CO3 and using the Henderson-Hasselbalch equation to calculate Ka1. Spectrophotometry, on the other hand, measures the absorbance of light at a specific wavelength to determine the concentration of H2CO3 and its dissociation products.
Applications of Ka1

The Ka1 value of H2CO3 has numerous applications, including:
- Calculating the pH of a solution containing H2CO3.
- Understanding the role of H2CO3 in natural processes, such as carbon dioxide sequestration in the ocean.
- Optimizing water treatment processes, such as water softening and pH adjustment.
FAQ Section
What is the significance of the Ka1 value?
The Ka1 value quantifies the strength of an acid, indicating its tendency to dissociate in water. It aids in predicting the pH of solutions and understanding the behavior of weak acids in various chemical processes.
How is the Ka1 value of H2CO3 determined?
Experimental methods, such as pH measurements using appropriate indicators, are employed to determine the Ka1 value of H2CO3 accurately. These methods involve measuring the pH of solutions containing known concentrations of H2CO3 and utilizing mathematical equations to calculate Ka1.
What are the applications of Ka1 in real-world scenarios?
Ka1 finds applications in diverse fields. It is used to calculate the pH of solutions, design buffers, and optimize processes involving weak acids like H2CO3. Additionally, Ka1 plays a crucial role in understanding carbon dioxide sequestration and water treatment, contributing to environmental sustainability.