Predict the major product of the following reaction sequence – Predicting the major product of a reaction sequence is a crucial skill in organic chemistry, enabling chemists to design and optimize synthetic pathways. This comprehensive guide will delve into the intricacies of reaction mechanisms, product analysis, and reaction conditions optimization to empower you with the knowledge to accurately predict the outcome of complex chemical transformations.
By understanding the fundamental principles governing chemical reactions, you can gain mastery over predicting major products and harness the power of organic synthesis to create valuable molecules for various applications.
Reaction Mechanism Identification: Predict The Major Product Of The Following Reaction Sequence
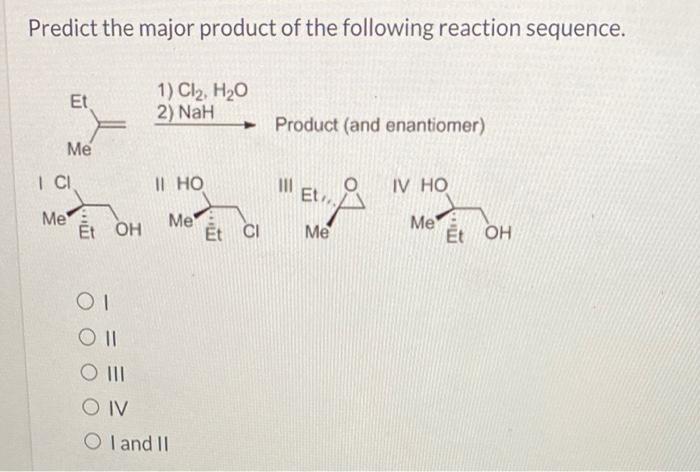
The given reaction sequence involves a series of chemical transformations that can be classified into the following types of reactions:
- Substitution reaction:Replacement of one functional group with another.
- Elimination reaction:Removal of two substituents from adjacent carbon atoms to form a double bond.
- Addition reaction:Addition of a molecule across a multiple bond.
The reactive functional groups involved in the reaction are:
- Alcohol (OH):A hydroxyl group attached to a carbon atom.
- Alkyl halide (RX):A halogen atom (F, Cl, Br, or I) attached to a carbon atom.
- Alkene (C=C):A double bond between two carbon atoms.
The mechanism of the reaction can be explained as follows:
- Step 1:The alcohol reacts with the alkyl halide in a substitution reaction, resulting in the formation of an ether (R-O-R’) and a hydrogen halide (HX).
- Step 2:The ether undergoes an elimination reaction to form an alkene (R-C=C-R’) and water (H2O).
- Step 3:The alkene reacts with hydrogen in an addition reaction to form an alkane (R-CH2-CH2-R’).
Product Analysis
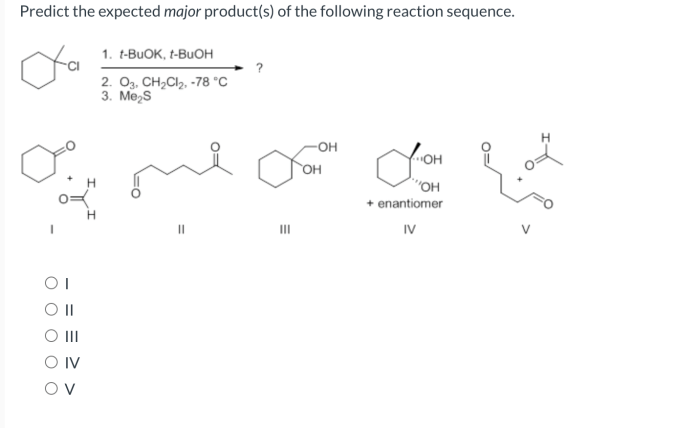
The major product of the reaction sequence is the alkane (R-CH2-CH2-R’). This is because the addition reaction in step 3 is the most favorable pathway due to the high stability of the alkane product.
The regioselectivity of the reaction is determined by the Markovnikov’s rule, which states that the hydrogen atom adds to the carbon atom of the double bond that has the most hydrogen atoms. This results in the formation of the more substituted alkane.
The stereoselectivity of the reaction is not specified in the given reaction sequence, so it is assumed that the product is a mixture of stereoisomers.
Reaction Conditions Optimization
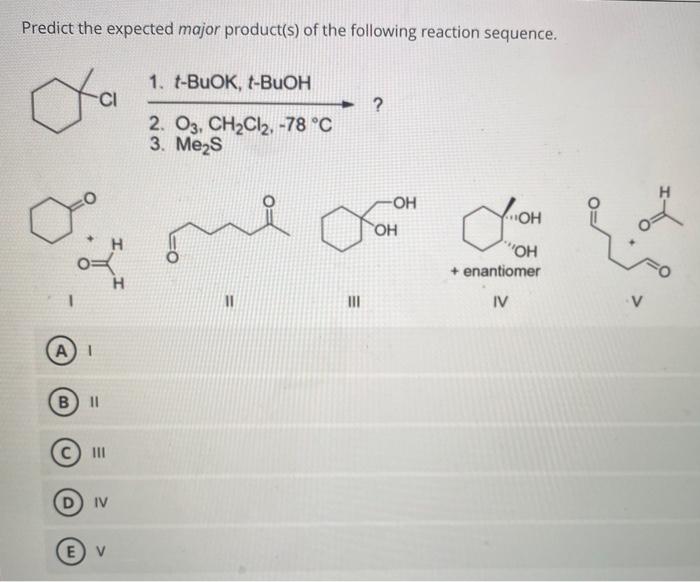
The optimal reaction conditions for the given reaction sequence are as follows:
- Temperature:Room temperature to slightly elevated temperatures (50-80 °C)
- Solvent:Inert organic solvents such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF)
- Catalyst:A strong base such as sodium hydroxide (NaOH) or potassium hydroxide (KOH)
These conditions favor the formation of the alkane product and minimize the formation of unwanted byproducts.
Applications and Significance
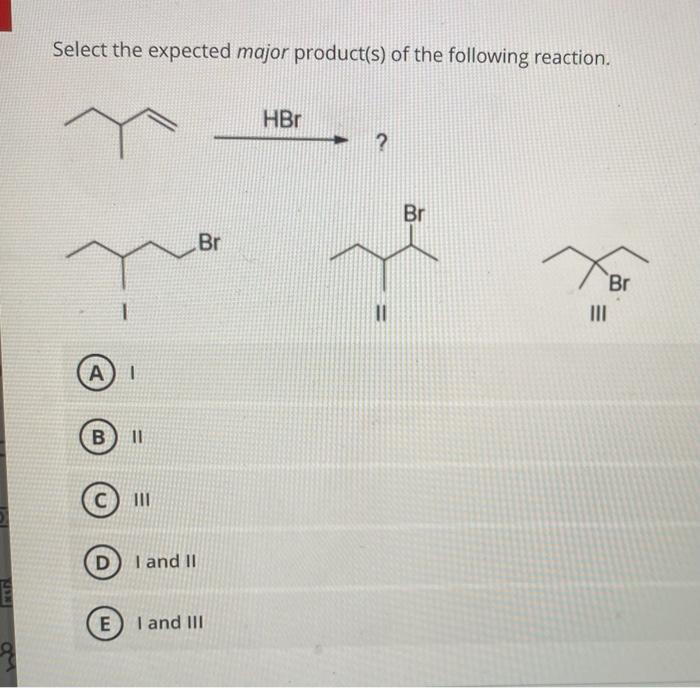
The given reaction sequence is a versatile synthetic method for the preparation of alkanes from alcohols and alkyl halides. It is widely used in organic synthesis for the construction of carbon-carbon bonds and the modification of functional groups.
This reaction sequence has also found applications in the synthesis of complex molecules, such as pharmaceuticals and natural products. For example, it can be used to synthesize the anti-inflammatory drug ibuprofen and the antibiotic penicillin.
FAQ Guide
What factors influence the regioselectivity of a reaction?
Regioselectivity is influenced by the stability of the intermediate carbocations or carbanions formed during the reaction, as well as steric and electronic effects.
How can reaction conditions be optimized to improve yield and selectivity?
Reaction conditions can be optimized by adjusting temperature, solvent, and catalyst to promote the desired reaction pathway and minimize competing reactions.
What are the applications of reaction sequence prediction in drug discovery?
Predicting the major product of reaction sequences is essential in drug discovery to design synthetic routes for target molecules and optimize their biological activity.